Why Is A Vinyl Proton Anisotropy

Electrons in π systems e g.
Why is a vinyl proton anisotropy. Propose possible structures for an unknown aromatic compound given its proton nmr spectrum other spectroscopic data such as a 13 c nmr or infrared spectrum or both. Undergraduate students combine molecular modeling and model building with diamagnetic anisotropy to explain why the singlet for the vinyl protons in the 1 h nmr spectrum of trans 1 2 dibenzoylethylene trans 1 4 diphenyl 2 butene 1 4 dione d 8 01 appears much farther downfield than that for the cis isomer d 7 14. So it s actually a lower chemical shift than a proton on a double bond. The shift for this proton turns out to be approximately two to 2 5.
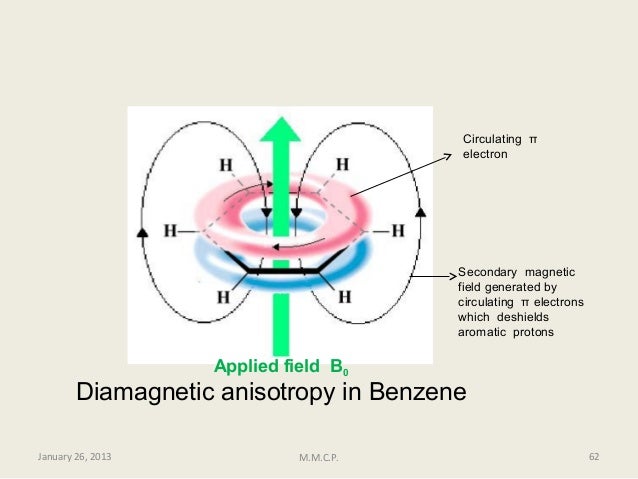
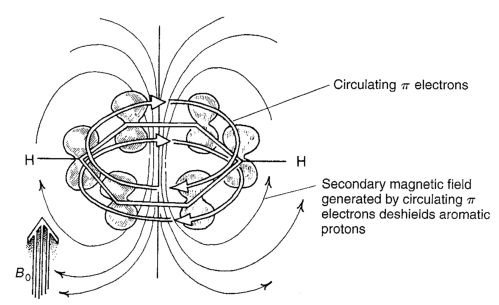
Aromatics alkenes alkynes carbonyls etc interact with the applied field which induces a magnetic field that causes the anisotropy as a result the nearby protons will experience 3 fields. Notice that the proton closest to the carbonyl group is at a higher chemical shift than the proton in cyclohexene 6 05 ppm for cyclohexenone vs. From these linear plots. In the trans isomer the vinyl protons are found in the deshielding regions.
Assuming that the proton chemical shifts were linearly dependent on the substituent electronegativity plus a constant shift arising from the diamagnetic anisotropy gave a value of 36 x10 6. The word anisotropic means non uniform so magnetic anisotropy means that there is a non uniform magnetic field. Nmr 1h chemical shifts alkenes c c anisotropy c c shielding. In the trans isomer the vinyl protons are found in the deshielding regions.
Now in the diagram consider the behaviour of the pi electrons in the applied magnetic field. In a similar manner zeil and buchert4 examined the proton chemical shifts of a variety of acetylenes and nitriles. But that s not what we observed. Now consider 2 cyclohexenone below.
And let s see if we can explain why. You know from physics when an electron encounters a magnetic field it sticks it little right hand out and moves. In nmr spectroscopy possibly the best example of anisotropy occurs with the benzene molecule in which the 6 pi electrons are delocalized and free to move around the aromatic ring. In this case a neighboring proton having a 1 2 spin shifts the resonance frequency of the proton being observed to a slightly higher value up to 7 hz and a 1 2 neighboring spin shifts it to a lower frequency.
Notice that the vinyl proton closest to the electronegative oxygen is pulled downfield i e higher ppm than the one further from the oxygen. Explain why signals resulting from the presence of aryl protons are found downfield from those caused by vinylic protons in a proton nmr spectrum. The applied field the shielding. 50 years but there is still controversy over the shielding effect of the double bond and no quantitative calculation of alkene proton chemical shifts has been given.
Undergraduate students combine molecular modeling and model building with diamagnetic anisotropy to explain why the singlet for the vinyl protons in the 1h nmr spectrum of trans 1 2 dibenzoylethylene trans 1 4 diphenyl 2 butene 1 4 dione d 8 01 appears much farther downfield than that for the cis isomer d 7 14.
.pdf+-+SumatraPDF_2012-12-20_00-45-54.png)









.pdf+-+SumatraPDF_2012-12-21_20-55-11.png)